
Clinical trials made simple
Scientifically engineered content + Digital, interactive technology.
Consenter delivers informed consent that speaks the participant’s language.

Customized content
Consenter’s content is customized to your clinical trial using evidence-based communication science strategies, including plain language, clear communication, and health literacy expertise.
Instead of reading long forms, patients engage with Consenter’s customizable on-screen avatar who explains the trial process and asks questions using plain language and optional knowledge assessments.


IRB required elements
Consenter delivers all IRB-required elements during informed consent—including the study’s purpose, study procedures, and potential risks and benefits of participation—ensuring consistent delivery across multiple sites and investigators.
Patient-centered
Consenter’s patient-centered approach and interactive activities enhance participant understanding of trial procedures so that participants who enroll are committed to the study.
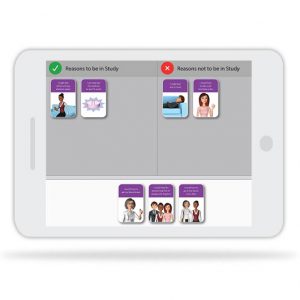
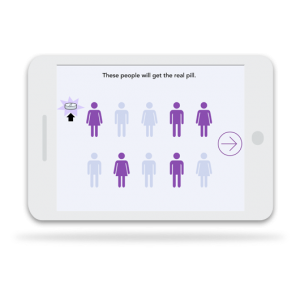
Workflow integration
Consenter is flexible and can be integrated into the workflow of your clinical trial. Deployment options range from on-site tablet-based apps to integration with existing eConsent platforms.
For remote enrollment, Consenter has partnered with THREAD Research to offer joint Consenter and eConsent services for virtual clinical trials.
Paper and eConsent Compatible
Consenter is IRB-compliant, compatible with both paper and eConsent processes, and focuses on getting the right participants enrolled in the right clinical trials.

Learn more about Consenter.
Get the right participants in your trial.

