Learn more about Consenter.
Get the right participants in your trial.

Features
Patient Centered
Content customized to your clinical trial using plain language and clear communication
IRB Required Elements
All IRB-required elements, including study purpose, procedures, and potential risks and benefits
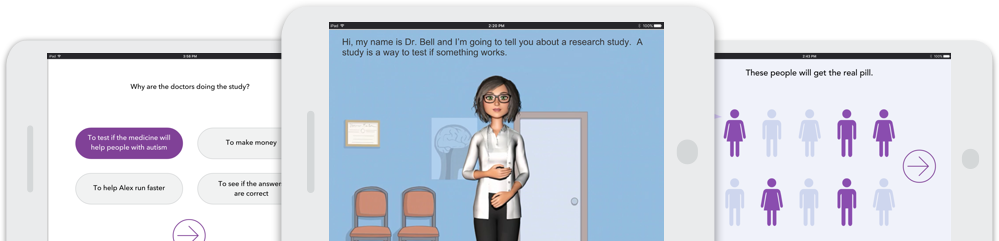
Interactive Technology
Patients engage with CONSENTER’s customizable on-screen avatar, who explains the trial process
Consistent Delivery
Ensure consistent consent delivery across multiple sites and investigators, reducing staff burden
Workflow Integration
Deployment options ranging from tablet-based apps to eConsent integration
Multiple Languages
Multiple languages available, including American Sign Language (ASL)
Learn more about Consenter.
Get the right participants in your trial.

